|
|

|
| In Saskatchewan, soil salinity affects several million acres of agricultural land to various degrees. In almost 600,000 acres, salinity restricts the growth of crops altogether. Farmers refer to saline soils by several names including Alkali and Burn-out. What is a saline soil? It is a soil that contains a high concentration of soluble salts such as sodium magnesium and calcium sulphates with lesser amounts of chlorides. Saline soils can be caused by contamination of normal soils by saline groundwater or spills or by development on saline parent material (Solonetzic Order) | ||||||||||||||||
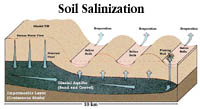
Salinization Process Saline soils are naturally occurring in Saskatchewan but can also be caused by poor irrigation practice. Saline soils result from the buildup of soluble salts in the soil. Ground water pressure maintains the water table close to the soil surface. Water moves by capillary action to the soil surface, bringing with it dissolved salts. When the water evaporates from the surface, these salts are left behind. Because evaporation exceeds precipitation in semi-arid climates, salts tend to build up particularly in the upper soil layers in low lying areas during dry periods. The excess salts (usually calcium sulphate) cause many types of crops to wither and die literally by starving them for water as a result of high osmotic pressure. Several factors cause salt to rise to the surface.
Saline soils have become an increasing problem over the years, due, in part, to traditional farming practices and construction activity which disrupts normal groundwater flow. Road construction and dam building are two activities associated with this problem. As water percolates through the soil, it dissolves the salts that are present in the soil. If the water carrying these salts move to the surface and evaporates, the salts are deposited at or close to the soil surface. A residue of dry salt is left once the water has evaporated. Saline soils are a problem because the salts prevent plant roots from making use of water in the soil. Plant roots absorb water from the soil through the process of osmosis. Osmosis moves water from an area of lower salt (higher water) concentration to an area of higher salt (lower water) concentration. The salt concentration inside a normal plant cell is about 1.5%, so that water moves into root cells. In saline soils, the concentration of salt is the soil water can rise above 1.5% and prevent osmosis from moving water into the roots. It may cause water to move out of the root, thereby dehydrating the plant. High salt content in the soil also causes nutrient deficiencies. Saline soils are characterized by high levels of salt and normal (neutral) pH levels. | ||||||||||||||||

Measuring Soil Salinity Soil salinity can be mapped in the field using an instrument called the EM38 earth conductivity meter. This instrument can be moved back and forth over the field and give a reading over the salinity electromagnetically. Soil salinity is usually determined as part of a routine soil test. Since salinity varies with depth it is very useful to send in soil samples that represent at least the surface soil (0-15cm), and sub soil (15-60cm). The results will then give us a salinity profile. The salts are extracted from soil by water in a saturation extract. The conductivity of the extract is then measured using a conductivity bridge. The more salt present the higher will be the conductivity. A value of 0-4 decisiemens/m (ds/m) is considered non saline to slightly saline. A value of 4-8 ds/m moderately saline and 8-16 ds/m severely saline. If the pH of the extract is > 8.5, and structure of the subsoil is hard and compacted suggesting a Bnt horizon or excess exchangeable sodium, then the ESP (Exchangeable Sodium Percentage) can be determined. This is the percentage of the soil's Cation Exchange Capacity occupied by sodium. An ESP of 15 % or greater is considered a sodic soil and needs special management practices. | ||||||||||||||||

Management of Saline Soils Saline soils must be managed with great care otherwise serious problems can persist for many years. The problem can spread rapidly and affect neighbour's fields or contaminate the local watershed or groundwater. Solutions to salinity probelems are often location specific and should be treated on a case by case basis for best results. Management programs that deal with salinity include:
Crops within each group are listed in order of increasing salt tolerance. If economic yields of annual crops cannot be maintained on saline land, then perennial forage production is the only management practice that can be recommended. As a general rule, mixtures of grasses and legumes are seeded so that at least some plant species will establish in even the most saline area and the more valuable species will grow on less saline portions of the field. The major reason for failure of forage crops to establish is seeding into seedbeds that are too loose and seeding depths that are too great. Forage crops, in general, are small seeded crops that require careful seedbed preparation and shallow seeding for successful establishment.
| ||||||||||||||||
Managing Sodic (Solonetzic) soils In managing these soils tillage operations and seeding must be done when the soil is at the correct moisture content. If the soil is worked when it is too wet, the structure breaks down and completely. If tillage is left too late, the soil bakes and it becomes almost impossible to get proper penetration of tillage implements. Deep plowing or deep ripping is a management practice designed to break up the compact Bnt horizon which resists penetration by plant roots. Usually done to a depth of 45-75 cm (18-30 in), this practice has proven beneficial in parts of Alberta and North Dakota and, more recently, in the Weyburn area of Saskatchewan. However, deep plowing at insufficient depth or where the subsoil is very saline or stoney can do more harm than good. Deep ripping needs special equipment (see image) pulled by a powerful tractor. Deep plowing is not a management or reclamation practice for saline soils. Alkalinity Salinity need not be confused with the term alkalinity. True Alkali soils rarely occur in Saskatchewan. They develop where the parent material is high in sodium. During the process of weathering, sodium is released from the mineral structure of shale. This process raises the pH (lowers the acidity) of the soil to the point where it becomes alkaline. The sodium ions cause the soil particles to lose their aggregation and pack tightly together. This packing closes the soil pores which then inhibits air and water movement. Alkali soils are characterized by a high pH (>8.5), high sodium concentrations and a dense soil layer that does not permit water to penetrate. Often the sodium and high pH causes some soil organic matter to dissolve which forms a black layer on the surface. These are called Black Alkali Soils. There are few true alkali soil areas in this province.
|

